My name is Megan, I am a fourth-year MSci Biochemistry and Genetics student at the University of Nottingham. This blog will serve as an online journal during my fourth-year project. I will review current science papers in the field as well as giving my own opinions on popular science. Stay tuned, there will be some exciting posts to follow!
My main scientific interest is in neurodegenerative disease, particularly Alzheimer’s disease and how cellular metabolism is implicated. Alzheimer’s disease is the leading cause of dementia in the elderly. It is associated with progressive decline of brain function which can affect memory and cognitive function. With an ageing population it is important for us to understand how this disease materialises and ways in which it can be treated.
There are two proteins that largely contribute to the pathology of the disease known as, amyloid beta and tau. The role of tau proteins is to stabilise microtubules within the distal region of neuronal axons. When the activity of tau kinases acting on tau is increased, the tau proteins misfold and aggregate forming neurofibrillary tangles inside neurons and less commonly in glial cells. Such tangles block nerve synapses and prevent nutrient transport between cells, causing neuronal death. The normal function of amyloid beta is not well understood. Pathological amyloid beta forms aggregates called amyloid fibres which are the main component of amyloid plaques, the pathological hallmark of Alzheimer’s disease. Unlike tau neurofibrillary tangles, the build-up of amyloid beta within amyloid plaques occurs outside the neurons.
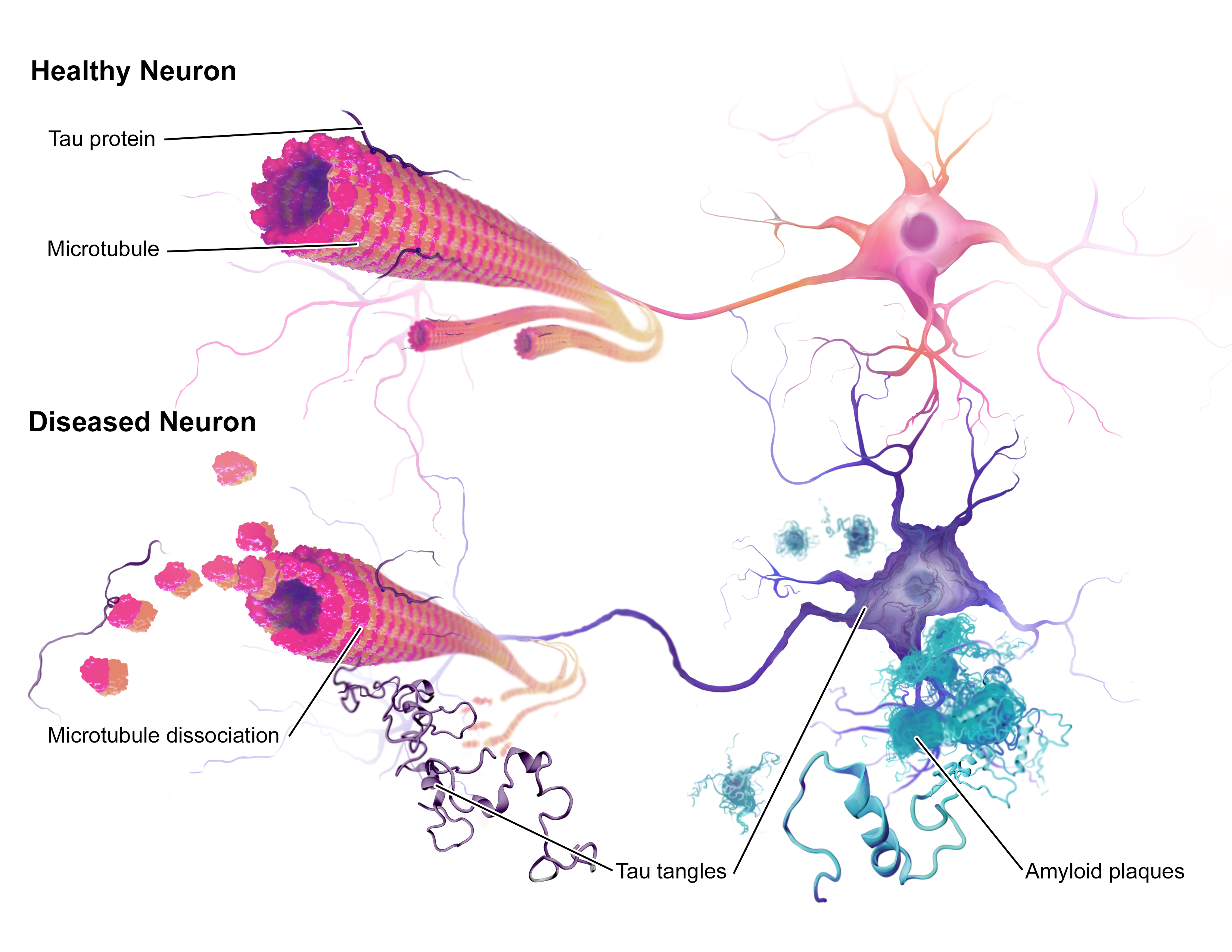
(Taken from: BruceBlaus [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)])
My fourth-year project involves using astrocytes which are a type of glial cell. Astrocytes are star shaped cells distributed throughout the brain and spinal cord supporting neurons within the central nervous system. Astrocytes connect neurons to the blood brain barrier and therefore serve as a mediator for transport of nutrients/molecules for signalling and overall neuron function. It was originally believed that they were merely supporting cells and while this is their primary function, other roles have come to light. For this reason, we are interested in whether they play a part in the pathology and progression of Alzheimer’s disease. Therefore, I am investigating the effects of amyloid beta on the metabolism and morphology of astrocytes.
Previous work by Garwood et al., showed that the level of interleukin 6 (IL-6) secretion from astrocytes was increased when incubated with amyloid beta. IL-6 is a cytokine known to activate the signalling molecule STAT3 through binding to its receptor on the surface of cells. This results in activation of the JAK-STAT pathway and modulation of STAT3 target genes in the nucleus.

(Taken from: Pharmstudice [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)])
During my project I am aiming to elucidate whether the incubation of astrocytes with amyloid beta will affect the signalling of STAT3 and whether this is likely to be due to the interplay of IL-6 and STAT3. My project will begin to address whether STAT3 signalling in astrocytes plays a role in Alzheimer’s disease and whether it affects the functioning of neurons.
My project began with optimising a method called immunocytochemistry (ICC). This involves using antibodies to stain specific proteins in cells for visualisation under a microscope. During the autumn term and beginning of the spring term I optimised ICC for the proteins STAT3 and GFAP. GFAP is a structural protein present in astrocytes and serves as a marker for the cells. I began the process optimising use of the fluorophore Alexa-488, a green fluorescent marker attached to an antibody. Although this worked, the amyloid beta I will be using has the same fluorophore attached to it, so I went on to optimise other antibodies. Next was Alexa-568, a red marker, which worked well for both STAT3 and GFAP. I then tried Alexa-405, a blue marker, however, it didn’t work after two attempts. This means that because STAT3 and GFAP worked well with the same antibody I will have to incubate my cells with amyloid beta, staining for STAT3 and GFAP separately instead of both at the same time with amyloid beta- Below is a gallery of some of the conditions I optimised during my preliminary work, click them to view them in full.
SVGA astrocytes stained for GFAP using primary antibody at 1/250 dilution and secondary antibody AlexaFluor 405 at 1/250 dilution. If this had been successful, staining would have appeared purple in the cytoplasm of the cells as a result of phalloidin a marker for the cytoplasm in red and the AlexaFluor being blue. 
SVGA astrocytes stained for GFAP using primary antibody at 1/250 dilution and secondary antibody AlexaFluor 568 at 1/250 dilution. 
SVGA astrocytes stained for GFAP using primary antibody at 1/250 dilution and secondary antibody AlexaFluor 488 at 1/250 dilution, amplified by Streptavidin tagged to Cy3. 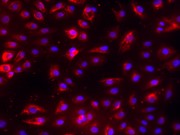
SVGA astrocytes stained for STAT3 using primary antibody at 1/250 dilution and secondary antibody AlexaFluor 568 at 1/250 dilution. 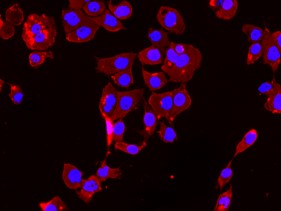
SVGA astrocytes stained for STAT3 using primary antibody at 1/250 dilution and secondary antibody AlexaFluor 488 at 1/250 dilution amplified by Streptavidin tagged to Cy3.
Now that the preliminary work is completed, I shall be moving on to the investigative portion of my project. First, I will be incubating my cells with the amyloid beta and performing ICC for STAT3 and GFAP with the conditions I determined before. This will be followed by analysis of my images which will involve:
- Counting the frequency of amyloid beta uptake
- Measuring the size of amyloid beta accumulation
- Measuring the length of cell filopodia (extensions from the cell body)
- Measuring the amount of fluorescence for STAT3 and GFAP
After this I will do an MTT assay and a glucose uptake assay. The MTT assay is a technique used to determine cell viability and the glucose uptake assay is a method for determining the metabolic activity of a cell.
Should STAT3 be affected by amyloid beta incubation and if there is enough time towards the end of my project, I would like to run an ELISA assay on the media that the astrocytes have been incubated in to see if IL-6 is present. If IL-6 is present, it may be the cause of alterations in STAT3 activity. If STAT3 is not affected by amyloid beta, an ELISA assay for a variety of cytokines could be used to determine alterations in the levels of other cytokines as a result of amyloid beta uptake.
That just about sums up my work so far and my plans for my project. If you have any questions or want to know more drop me a comment below 🙂
