A few weeks ago I was shown a really interesting paper that I just had to write about on my blog. Stephen Dominy et al., have evidence to suggest that bacteria in the mouth are related to the pathology of Alzheimer’s disease. The bacteria in question: Porphyromonas gingivalis, is the key pathogen responsible for chronic periodontitis, more commonly known as gum disease.
As mentioned in one of my previous posts, the proteins amyloid beta and tau are involved in the pathology of Alzheimer’s disease. Since the 1980’s, the leading hypothesis for the pathology of Alzheimer’s disease known as the “amyloid hypothesis”, proposes that the condition is caused by the improper function of these proteins which accumulate in the brain. This occurs outside cells in the case of amyloid-beta and inside cells for tau.
Recently gum disease and infection with P. gingivalis have been identified as significant risk factors for developing amyloid beta plaques, dementia and Alzheimer’s disease (See my introductory post for more information about Alzheimer’s disease). One study of patients with both Alzheimer’s disease and gum disease reported decline in cognition over a 6-month period, compared to those without gum disease. This led to investigation of possible mechanisms underlying these findings. P. gingivalis produces protein-degrading enzymes known as gingipains which are essential for the survival and pathogenic capabilities of P. gingivalis. Due to the emerging evidence of P. gingivalis being involved in Alzheimer’s disease, Dominy et al., hypothesised that:
P. gingivalis infection acts in Alzheimer’s disease pathogenesis through the secretion of gingipains to promote neuronal damage.
They examined samples of Alzheimer’s disease brain tissue as well as that of neurologically normal individuals. Of the 54 Alzheimer’s disease samples taken from the hippocampus, the region of the brain important for memory, they discovered that P. gingivalis was present in over 90% of the samples. They also found a correlation between the amount of P. gingivalis in Alzheimer’s disease samples and the protein tau. Alzheimer’s disease is also associated with degradation of the grey matter of the cerebral cortex. For this reason the cerebral cortex of three Alzheimer’s disease brain samples was examined, with genetic material from P. gingivalis being detected.
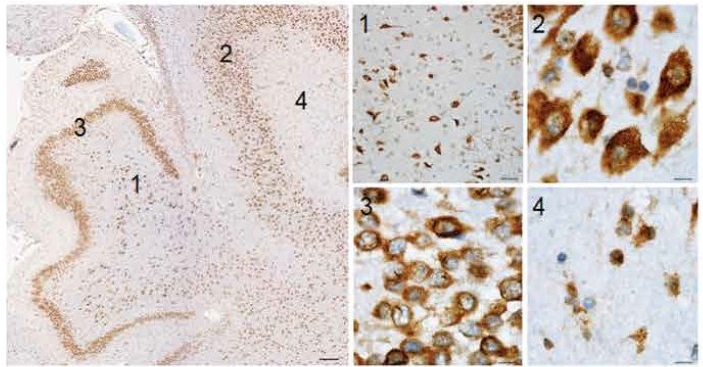
Representative images of the hippocampal brain region stained for gingipains in a 63-year-old patient.
The hippocampus shows abundant gingipains in the hilus (1), CA3 pyramidal layer (2), granular cell layer (3), and molecular layer (4). High-magnification images from the indicated areas (1 to 4) exhibit a granular staining pattern consistent with P. gingivalis intracellular infection. Scale bars, 200 micrometres (overview), 50 micrometres (1), and 10 micrometres (2 to 4).
(Figure and legend modified from Figure 2: Dominy et al).
The team when on to test the cerebrospinal fluid of 10 patients diagnosed with probable Alzheimer’s disease who presented mild to moderate cognitive impairment. For these patients, P. gingivalis was present in 7 of the 10 samples. This combined with the fact that P. gingivalis associates with tau, led to them determining whether tau is a target of gingipain degradation. The results showed that the gingipains cleaved tau into fragments which have previously been found to be involved in tau tangle formation. These findings led to the proposal that P. gingivalis does not enter the brain as a result of Alzheimer’s disease onset but it could be the cause of it. This indeed appeared to be the case when mice were infected with P. gingivalis resulting in gum disease. This led to brain infection, amyloid production, tau tangle formation and neural damage as seen in Alzheimer’s disease.
This research is vital in understanding which groups of people are more susceptible to Alzheimer’s disease, one of which is individuals with Down syndrome. Within this group there is a high prevalence of dementia with Alzheimer’s-type pathology. This is believed to be due to the fact that the amyloid precursor protein gene which gives rise to amyloid beta is present on chromosome 21 and this chromosome is triplicated in Down syndrome. The team point out that the occurance of P. gingivalis is found to be significant in the mouths of Down syndrome patients beginning in early childhood compared to age-matched individuals without Down syndrome, indicating that P. gingivalis is present at abnormal levels in Down syndrome patients. The susceptibility of Down syndrome patients to P. gingivalis is not yet clear but is suggested to be due to having weakened immune systems.
Future work will need to identify the route of entry into the brain by P. gingivalis. However, the group does propose that once in the brain it spreads from one neuron to the next, which may be the cause of the tau pathology in Alzheimer’s diseased brains, which had already been suggested to occur in this manner. Casey Lynch a member of the team describes their hypothesis that P. gingivalis infection is the cause rather than a result of Alzheimer’s disease as being,
A universal hypothesis of pathogenesis, fully explaining the cause of Alzheimer’s disease.
This paper was certainly very exciting to read. The concept of the “amyloid hypothesis” is well-supported within the scientific community. For this new study to propose that amyloid beta and/or tau dysfunction is not the cause but one of the effects of the onset of Alzheimer’s disease, is truly ground-breaking.
In the latter part of their paper they describe methods for blocking gingipain activity. This involved administering small-molecular inhibitors to mice with Alzheimer’s disease pathology resulting from P. gingivalis infection. The results of which showed reduced infection, prevention of amyloid production and lowered inflammation. The small-molecular inhibitors are currently being trialled in human clinical studies for Alzheimer’s disease. If these studies have positive outcomes then this group may have just found the cause and cure of Alzheimer’s disease!
